Introduction: The Importance of Compliance in Manufacturing Industry
Compliance in the manufacturing industry is a foundation for operational integrity, product quality, and market trust. As global standards evolve and consumer expectations rise, production compliance plays a central role in ensuring that every step of the manufacturing process meets legal, ethical, and safety benchmarks.
For any compliance-focused manufacturing company, the risks of overlooking regulations are significant: disrupted operations, financial penalties, reputational damage, and even product recalls. More than ever, staying compliant means staying competitive. This is how reality looks today.
Building strong compliance practices enables manufacturers to avoid costly missteps, improve efficiency, and foster a culture of accountability across teams. From environmental mandates and safety standards to data integrity and quality control, modern compliance for manufacturing companies is complex - but critical.
This article outlines what production compliance really entails, the key regulations to follow, common pitfalls to avoid, and how new technologies like AI are transforming the compliance landscape in 2025 and beyond.
Understanding Production Compliance: What It Means for Your Operations
With the stakes this high, it’s essential to understand what compliance actually looks like on the factory floor and how it impacts day-to-day operations.
Production compliance in the manufacturing industry is a structured system that ensures every stage of the manufacturing process aligns with legal obligations, industry expectations, and standards that are created for such industry manufacturing. For any compliance-driven manufacturing company, it serves as both a shield against risk and a foundation for long-term operational excellence.
At its core, production compliance consists of three critical layers: regulatory compliance, industry standards compliance, and internal policy compliance.
Regulatory Compliance
Regulatory compliance forms the legal backbone. It includes mandatory requirements set by national and international authorities such as the FDA, OSHA, EPA, or EU regulatory bodies. These rules govern everything from equipment safety and environmental protection to material handling, labeling, and traceability. Non-compliance in this area can result in severe consequences - fines, product recalls, halted operations, or even permanent business damage. For manufacturers, adhering to these regulations is essential not only for staying operational but also for maintaining market credibility.
Industry Standards Compliance
Complementing these legal mandates is industry standards compliance that serves as a mark of operational maturity and global competitiveness. Standards like ISO 9001 (quality management), ISO 14001 (environmental management), GMP (Good Manufacturing Practice), and HACCP (food safety) provide detailed frameworks for quality assurance, risk management, and process control. Certification to these standards signals that a manufacturing company meets internationally accepted benchmarks, unlocking access to new markets and fostering stronger relationships with clients, regulators, and partners.
Internal Policy Compliance
The third layer, internal policy compliance, is shaped by the company itself. These are organization-specific rules, procedures, and values that often go beyond legal and industry requirements. Internal policies may include advanced safety protocols, environmental goals, supply chain ethics, proprietary quality controls, or continuous improvement methodologies. By aligning internal operations with strategic goals, companies strengthen accountability, promote consistency, and build a resilient compliance culture that supports both growth and audit readiness.
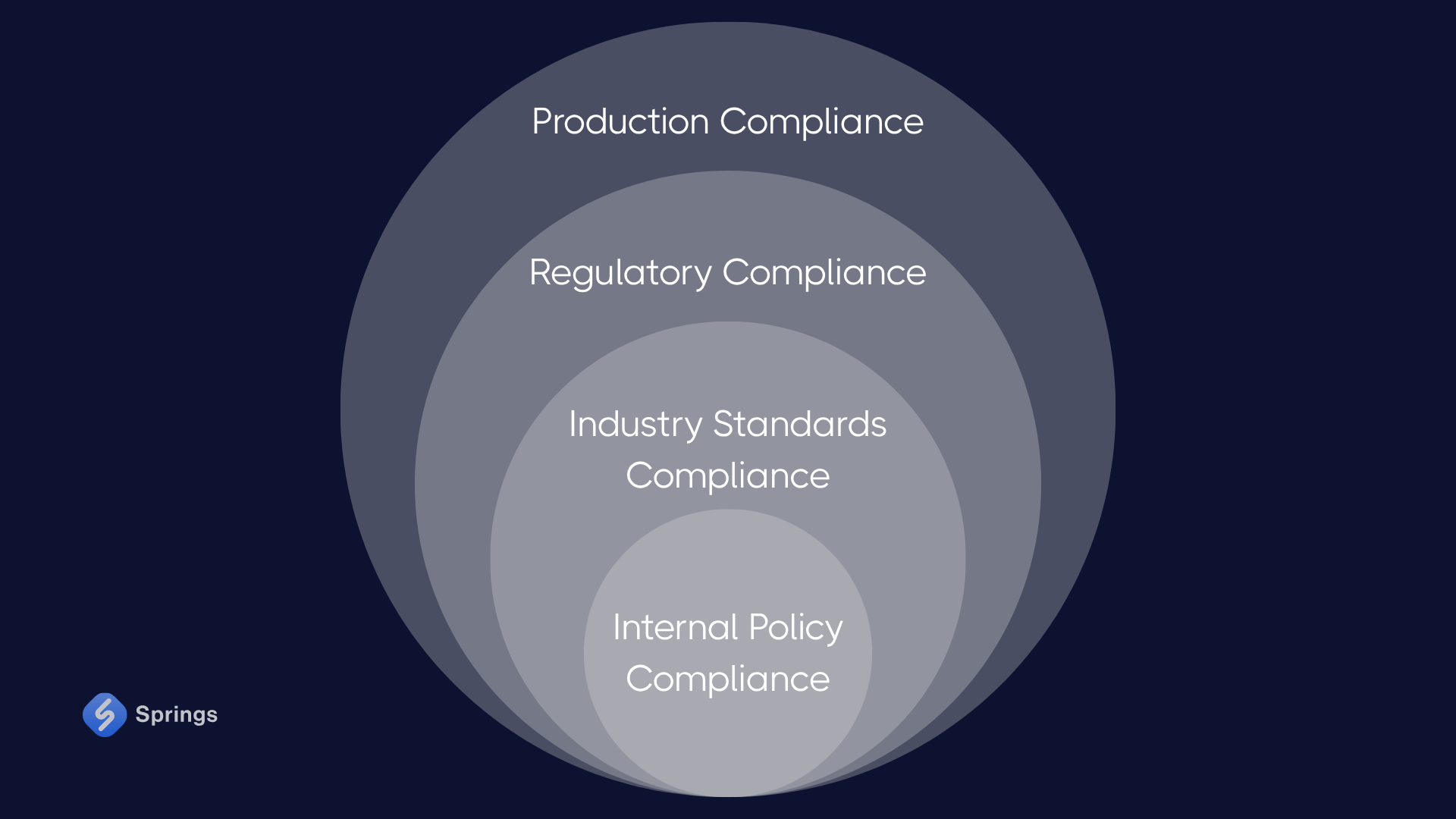
Together, these three types of compliance create a unified framework for production compliance, ensuring that a manufacturing company meets minimum standards and excels in delivering safe, reliable, and regulation-compliant products. In today’s high-stakes environment, this layered approach is essential for managing risk, maintaining operational stability, and securing long-term success.
Key Regulations Every Manufacturing Company Must Follow
Compliance in the manufacturing industry is regulated by many strict national laws, industry-specific policies, and global frameworks. To build a reliable production compliance strategy, manufacturing companies must understand how these regulations apply in their target markets and sectors. Below is a closer look at the most critical regulations by region, along with real-world examples to show how they translate into operational requirements.
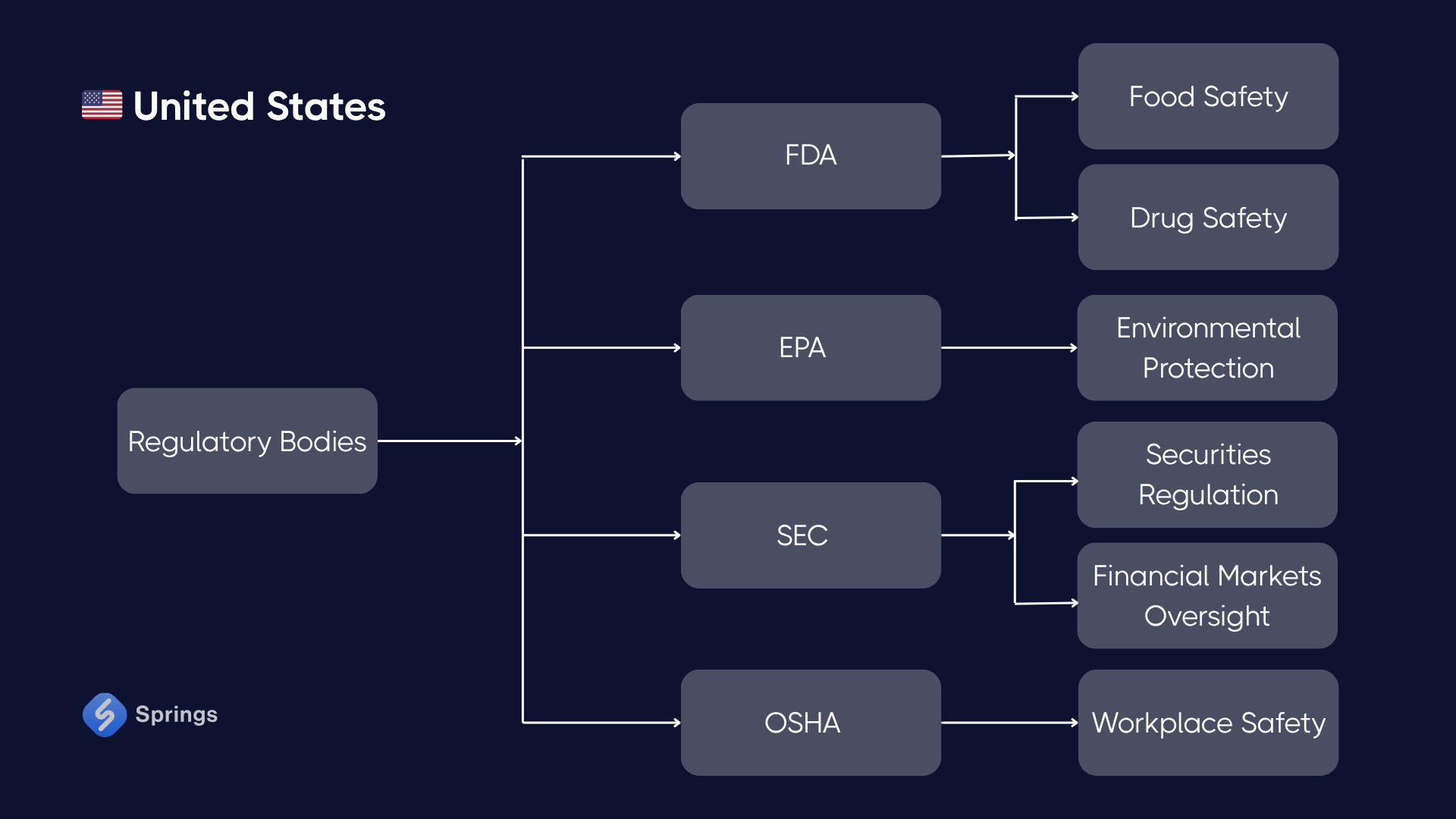
🇺🇸 United States
Key Regulations:
- FDA cGMP (21 CFR Parts 210–211): Applies to pharmaceutical, biotech, food, and cosmetics manufacturers. Requires documented processes, validated systems, cleanliness controls, and robust quality oversight.
- OSHA Process Safety Management (29 CFR 1910.119): For manufacturers using toxic or reactive chemicals, mandates hazard analysis, mechanical integrity programs, and employee training.
- OSHA General Industry Standards (29 CFR 1910 Subpart I – Personal Protective Equipment): Mandates the use of appropriate PPE (e.g., gloves, eye and face protection, respiratory gear) and regular hazard assessments.
- EPA Clean Air Act / Clean Water Act: Controls emissions and effluent discharge; relevant for metal processing, chemical, and energy-intensive industries.
- CPSC (Consumer Product Safety Commission): Ensures the safety of consumer goods through product testing, warnings, and labeling requirements.
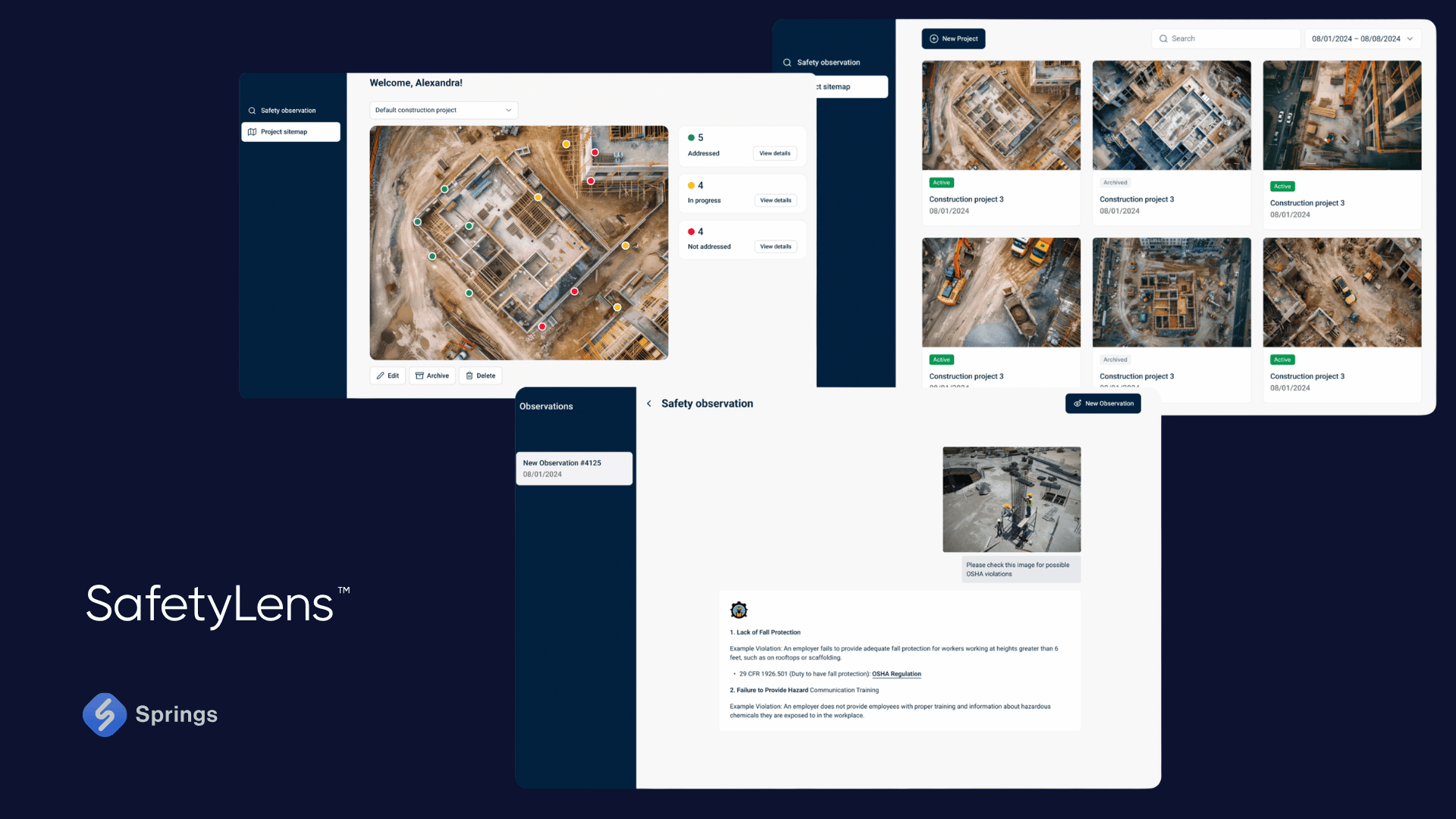
Case – AI-Enhanced OSHA Compliance in Manufacturing:
A U.S.-based mid-sized manufacturing firm partnered with Springs to deploy SafetyLens™, an AI-powered platform designed to enhance compliance with OSHA workplace safety standards. Specifically targeting 29 CFR 1910 Subpart I (PPE) and 1910.22 (walking-working surfaces), the system uses computer vision to detect real-time non-compliance issues - like employees not wearing helmets or safety goggles, blocked emergency exits, and improperly stored equipment.
Using machine learning models trained on job site imagery, SafetyLens™ automatically flags violations, sends alerts to safety officers, and generates compliance logs aligned with OSHA documentation standards. This not only reduced incidents and violations but also helped the company streamline reporting during audits and avoid potential fines for repeated safety breaches.
By embedding this AI layer into their production compliance framework, the company turned reactive safety enforcement into a predictive, ongoing compliance strategy - one that aligned directly with federal regulatory mandates and supported continuous improvement.
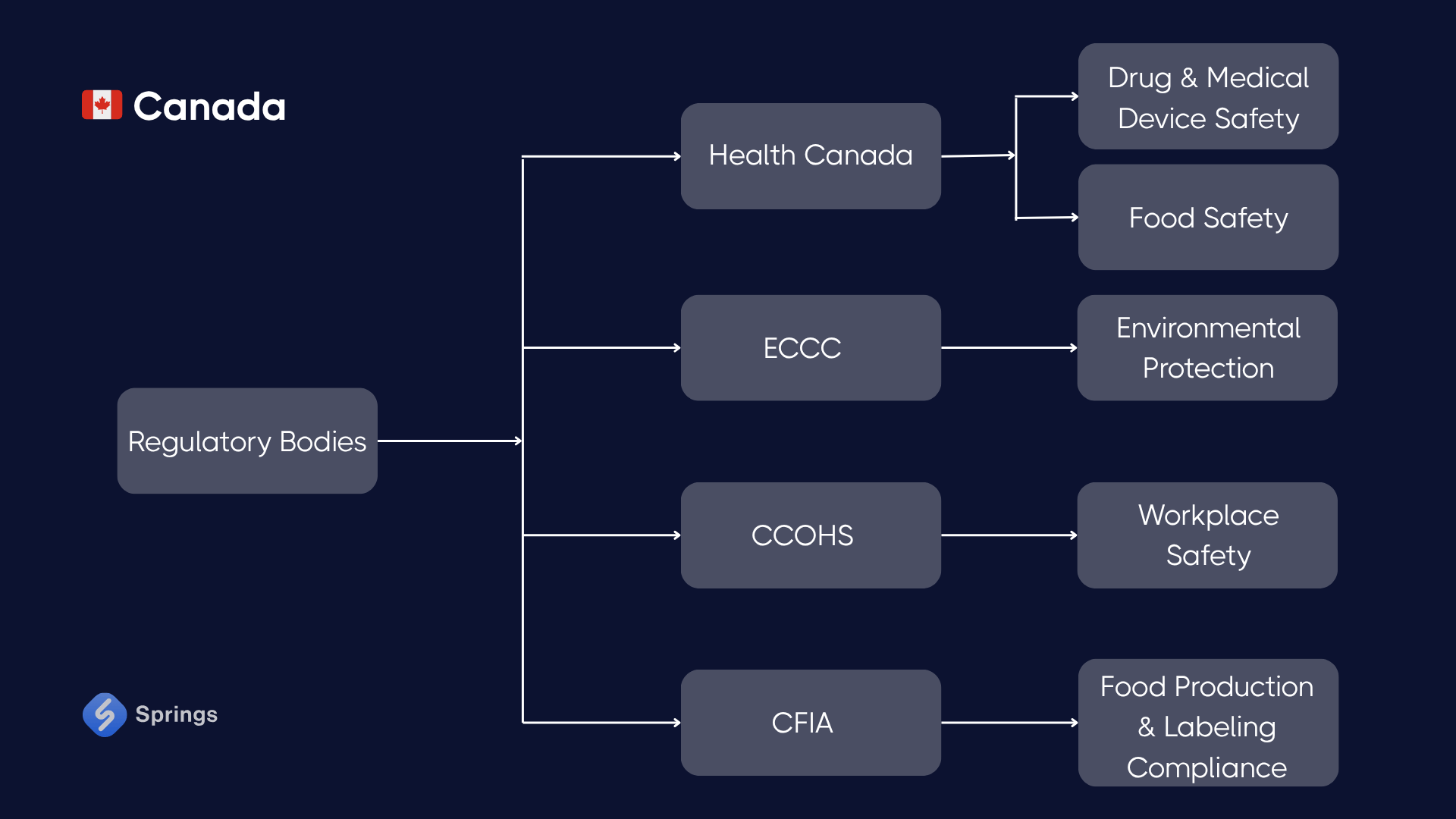
🇨🇦 Canada
Key Regulations:
- Safe Food for Canadians Regulations (SFCR): Requires preventive controls, traceability, and recall systems.
- Hazardous Products Act (aligned with GHS): Mandates classification and labeling of chemicals.
- Environmental Protection Act: Governs emissions, storage, and disposal of industrial waste.
Case – Snack Foods Manufacturer (Ontario):
A facility producing nut-based snacks must implement a HACCP-based plan under SFCR, with sanitation logs, allergen separation procedures, and rapid traceability. Products exported to the U.S. are also subject to FDA FSMA rules, requiring dual compliance systems.
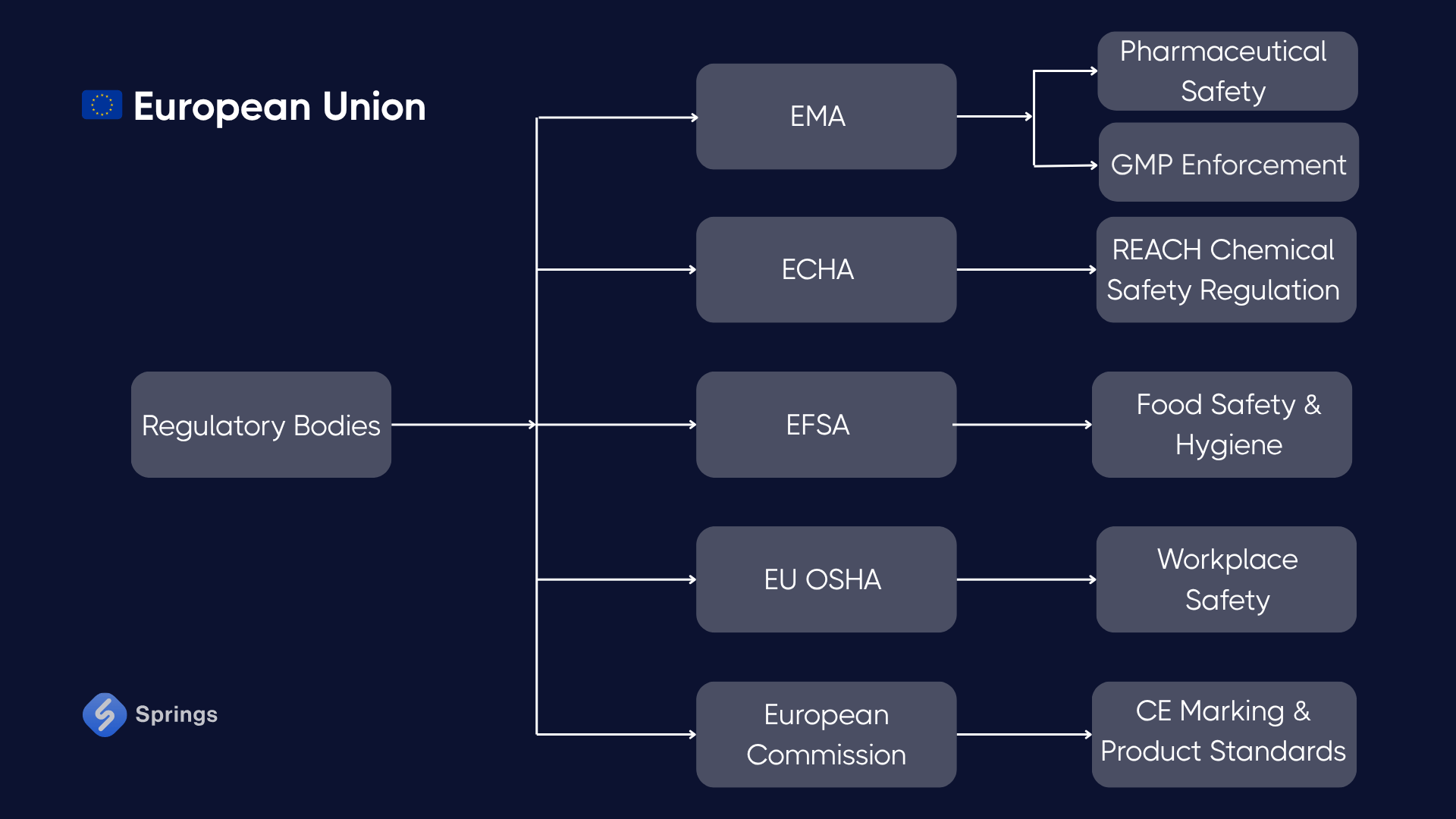
🇪🇺 European Union
Key Regulations:
- REACH Regulation (EC 1907/2006): Requires registration, risk assessment, and communication for chemicals over 1 ton/year; essential for manufacturers and importers.
- CE Marking & Machinery Directive (2006/42/EC): Applicable to most industrial and electrical equipment; requires conformity assessments and technical documentation.
- PED (Pressure Equipment Directive): Sets standards for the design and fabrication of pressure vessels and piping.
- Corporate Sustainability Due Diligence Directive (CSDDD): Enforces environmental and human rights due diligence for large companies and their supply chains.
- Cyber Resilience Act (CRA): Introduces mandatory cybersecurity design and post-market obligations for digital products.
Case – Machinery Manufacturer (Germany):
A CNC machine producer exports to France and Italy. To obtain CE marking, the company complies with the Machinery Directive, prepares a technical file, and conducts risk analysis under ISO 12100. A newly launched IoT-enabled model is redesigned to comply with the CRA, ensuring software integrity, update policies, and vulnerability disclosure mechanisms.
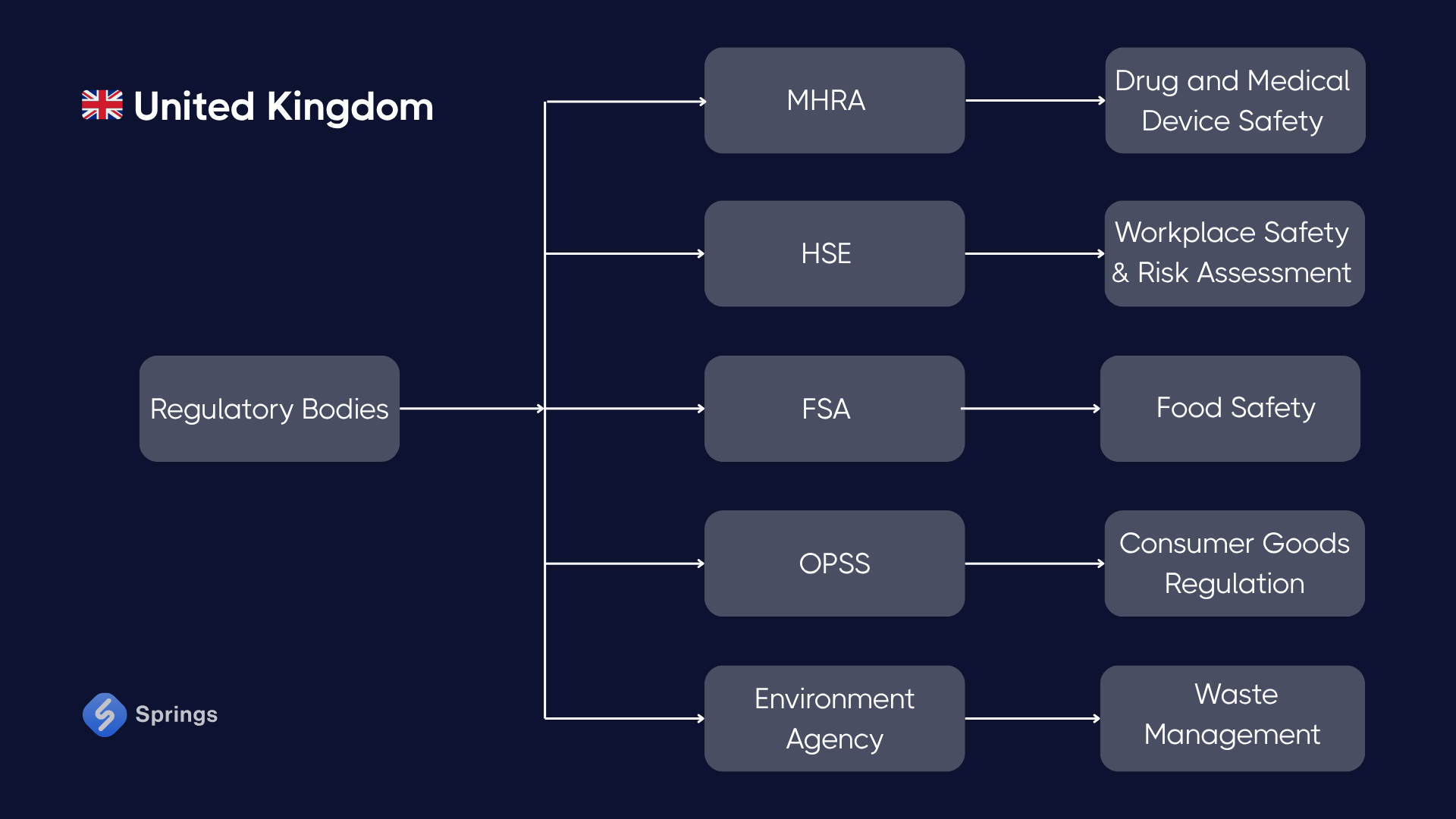
🇬🇧 United Kingdom
Key Regulations:
- UKCA Marking: Post-Brexit, products require UKCA instead of CE for access to the British market.
- Health and Safety at Work Act (1974): Core legislation governing worker safety and employer duties.
- COSHH (Control of Substances Hazardous to Health): Requires assessment and control of chemicals in the workplace.
Case – Coatings Manufacturer (Manchester):
A company producing industrial paints handles solvents and must follow COSHH by implementing local exhaust ventilation, training staff in PPE use, and keeping up-to-date SDSs. UKCA marking is required on automated mixing machines sold domestically, with technical documentation stored for 10 years.
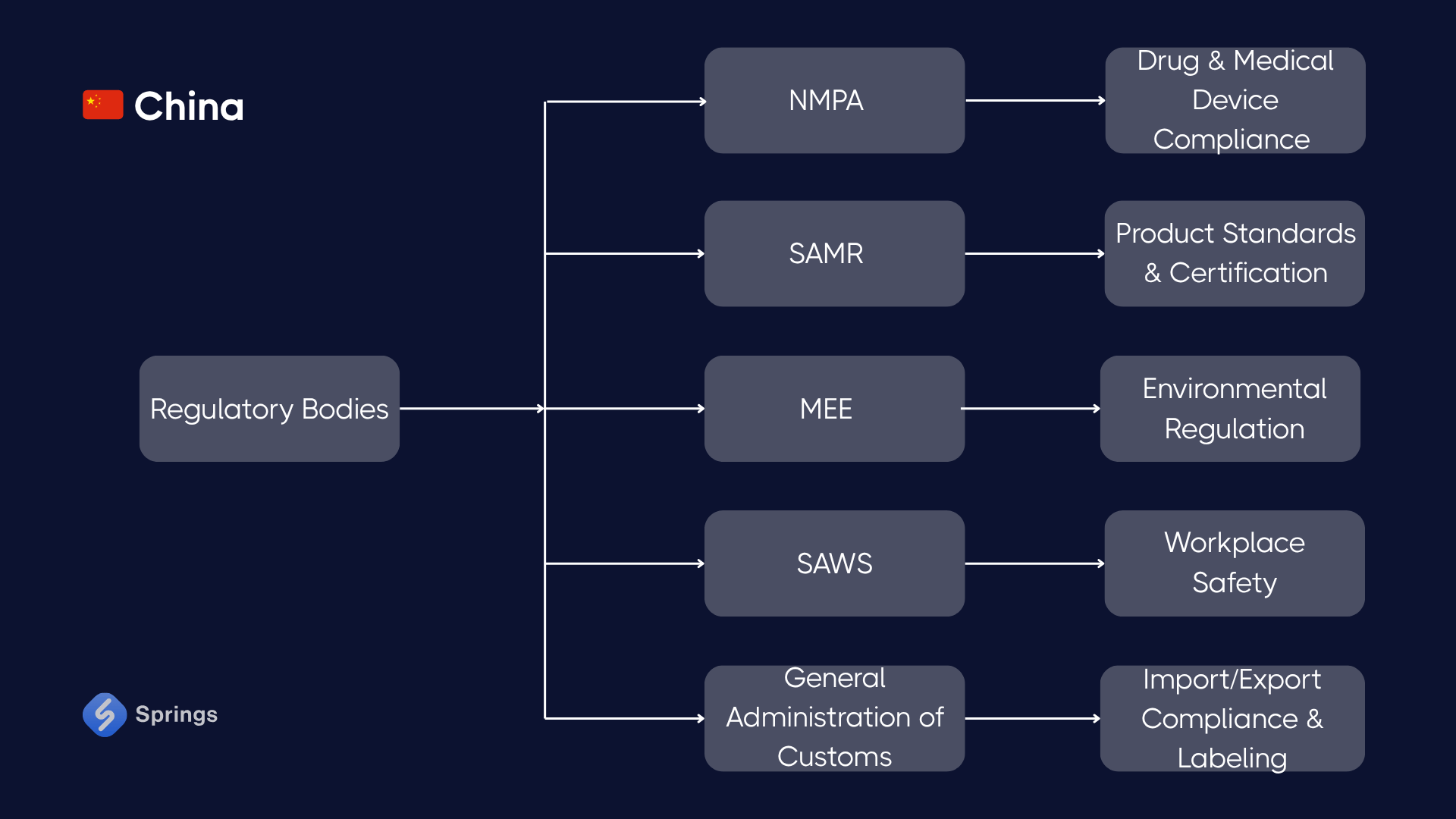
🇨🇳 China
Key Regulations:
- China RoHS II: Restricts use of six hazardous substances (lead, mercury, cadmium, etc.) in electrical/electronic products.
- GB Standards: National compulsory standards across mechanical safety, environmental performance, and labeling.
Case – Electronics Manufacturer (Shenzhen):
A smartphone assembler must declare compliance with China RoHS II, label devices with the EFUP symbol, and disclose substance content in manuals. Additional GB standards apply to fire resistance, battery testing, and electromagnetic compatibility.
Cross-Border Standards and Sector Overlays
Global standards often apply across regions and help unify production compliance efforts:
- ISO 9001: Quality management systems
- ISO 14001: Environmental management
- IATF 16949: Automotive industry compliance
- GMP (WHO/EU/FDA): Pharmaceutical production standards
- HACCP: Food safety risk controls
- ISO 45001: Occupational health and safety
Example – Automotive Supplier (Mexico):
A Tier 1 supplier must meet IATF 16949, ISO 14001, and local Mexican labor laws. Internal audits, FMEA risk assessments, and supplier scorecards form part of a multi-layered compliance framework. To export to the EU, REACH and ELV (End-of-Life Vehicle Directive) also apply.
For a manufacturing company operating across borders, production compliance means more than meeting baseline regulations. It requires dynamic alignment with national laws, sector standards, and customer expectations - backed by rigorous documentation, training, and audit readiness.
Common Challenges in Compliance for Manufacturing Company and How to Overcome Them
While regulatory frameworks set the foundation, it’s the day-to-day execution where most compliance in manufacturing industry efforts are tested. Even the most well-intentioned companies face operational, technical, and strategic hurdles that can compromise production compliance - especially when navigating multiple jurisdictions.
1. Navigating Complex, Multi-Jurisdictional Regulations
Challenge: A manufacturing company operating across borders must comply with overlapping and sometimes conflicting regulations. For example, a medical device firm exporting to both the EU and the U.S. must align with FDA 21 CFR Part 820 and the EU MDR, which differ in documentation, risk classification, and post-market surveillance requirements.
How to Overcome It:
- Establish a regulatory intelligence team to track country-specific updates.
- Use a compliance management system (CMS) to centralize global documentation and workflows.
- Map product-specific requirements across all target markets during product design.
2. Inconsistent Documentation and Recordkeeping
Challenge: Incomplete batch records, missing calibration logs, or inconsistent labeling can result in failed audits or product recalls. This is particularly risky in industries governed by GMP, HACCP, or IATF 16949.
How to Overcome It:
- Digitize documentation with electronic batch records (EBR) or document control systems.
- Standardize templates across regions to align with ISO 9001 or FDA requirements.
- Conduct regular internal audits to spot gaps before regulators do.
3. Supply Chain Non-Compliance
Challenge: Many compliance risks originate upstream. For instance, under REACH and CSDDD (EU), companies are now responsible for ensuring their suppliers meet environmental and human rights standards. A non-compliant raw material supplier can jeopardize an entire production batch.
How to Overcome It:
- Implement supplier qualification and monitoring programs.
- Require vendors to provide compliance declarations, safety data sheets (SDS), or material traceability certificates.
- Adopt platforms like IMDS (automotive) or Sedex (ethical sourcing) for supply chain visibility.
4. Lack of Workforce Training and Awareness
Challenge: Operators unaware of updated SOPs, labeling changes, or hazard handling procedures are a major source of non-compliance. In countries like India or Mexico, where regulations are rapidly evolving, local facilities may fall behind on training.
How to Overcome It:
- Develop role-specific training modules, updated quarterly or when regulations change.
- Use learning management systems (LMS) to track completion and competence.
- Incorporate compliance KPIs into HR and performance reviews.
5. Rapid Regulatory Changes and Unclear Guidelines
Challenge: New rules - like the EU’s Cyber Resilience Act - often lack full implementation guidelines or are released with tight deadlines, leaving manufacturers unsure how to respond.
How to Overcome It:
- Join industry associations or standards committees for early access to updates and interpretations.
- Maintain agile compliance protocols that allow quick adaptation without disrupting production.
- Work with regulatory consultants for gap analyses and implementation roadmaps.
6. Technology Integration Gaps
Challenge: Legacy systems often can’t support compliance features like traceability, version control, or real-time reporting. This creates blind spots in production compliance, especially in FDA, GFSI, or ISO-regulated environments.
How to Overcome It:
- Invest in ERP or MES systems with built-in compliance modules (e.g., SAP, Plex, MasterControl).
- Automate CAPA, audit tracking, and change control workflows.
- Leverage AI tools for real-time deviation alerts and predictive risk management.
7. Audit Fatigue and Resource Constraints
Challenge: Especially in regulated industries like pharma or aerospace, multiple audits from regulators, clients, and certifiers can overwhelm internal teams, leading to burnout or oversights.
How to Overcome It:
- Schedule mock audits to maintain a constant state of readiness.
- Cross-train staff on audit procedures and documentation access.
- Use compliance dashboards to surface red flags before external reviews.
Manufacturing companies that view compliance not as a one-time obligation but as a strategic capability are better equipped to overcome these challenges. Building resilient systems, adopting the right technologies, and fostering a culture of accountability are essential steps toward sustainable production compliance - no matter the geography.
Building a Robust Compliance Program in the Manufacturing Industry
Overcoming compliance challenges isn’t enough - manufacturing companies need structured, future-proof systems that embed compliance into every layer of their operations. A fragmented or reactive approach exposes organizations to unacceptable risks. What’s required is a deliberate, end-to-end compliance program tailored to the complexity of modern manufacturing environments.
A strong compliance program is a living framework that supports continuous adherence to evolving regulatory demands. Whether you're operating domestically or across borders, production compliance must be embedded in your workflows, culture, and systems.
Below are the essential pillars of a robust compliance strategy for any manufacturing company:
1. Regulatory Mapping by Market and Sector
Every compliance program must begin with a detailed understanding of applicable regulations, which can vary by product type and geographic scope.
- United States: FDA cGMP (pharma/biotech), OSHA (safety), EPA (environment), CPSC (consumer goods)
- European Union: REACH, CE marking (Machinery Directive), PED, CSDDD, CRA (connected devices)
- China: China RoHS II, GB standards (national technical specs)
- Canada & UK: SFCR, UKCA, COSHH, WHS regulations
Best Practice: Create a centralized regulatory database linked to SKUs and product categories. Assign ownership by region to ensure updates are tracked and implemented.
2. Integrated Policy and SOP Framework
To support production compliance, companies need formalized policies and SOPs aligned with both legal and internal requirements. These documents must be version-controlled and accessible across teams.
Implementation Tactics:
- Align SOPs with ISO standards (e.g., ISO 9001 for QMS, ISO 14001 for EMS)
- Translate documents for multilingual facilities
- Synchronize SOP updates with training modules
3. Technology Infrastructure for Compliance Execution
Outdated systems often lead to gaps in traceability, documentation, and reporting - all of which are critical in regulated industries.
Core Systems:
- ERP (Enterprise Resource Planning): Supports document control, supplier compliance, and real-time production monitoring
- MES (Manufacturing Execution Systems): Enables batch control, shop-floor data collection, and deviation tracking
- QMS (Quality Management Systems): Automates CAPA, change control, internal audits, and compliance reporting
Note: In jurisdictions like the U.S. and EU, these systems must comply with standards like FDA 21 CFR Part 11 (electronic records) or ISO 13485 (medical devices).
4. Cross-Functional Ownership and Accountability
Compliance for manufacturing companies fails when it’s siloed. Successful programs embed compliance responsibilities into operations, quality, legal, and procurement departments.
Best Practices:
- Assign regional compliance leads
- Set up a compliance steering committee that meets quarterly
- Integrate compliance KPIs into departmental scorecards
5. Training, Competency, and Cultural Adoption
Compliance in manufacturing industry settings requires that workers - from the shop floor to senior management - fully understand their roles in maintaining standards.
Effective Strategies:
- Use LMS platforms to deliver, track, and audit training
- Tailor modules by role (e.g., machine operator vs. quality auditor)
- Offer refresher courses post-regulatory updates
6. Risk-Based Monitoring and Internal Auditing
Regular audits ensure that what’s on paper aligns with what happens in practice. A proactive, risk-based approach helps identify weak points before external audits do.
Audit Structure:
- Frequency based on risk level and regulation (e.g., monthly GMP line audits, quarterly ISO inspections)
- Randomized walkthroughs and batch record reviews
- Use of automated audit logs in QMS or CMS platforms
7. Continuous Improvement and Regulatory Foresight
Compliance isn’t static. New regulations - like the EU Cyber Resilience Act - require fast adaptation.
Action Points:
- Monitor regulatory bodies (FDA, EMA, CDSCO, MHRA, etc.)
- Benchmark audit results and CAPA data to identify patterns
- Participate in global standardization groups (ISO, ICH, ASTM)
A truly robust compliance program positions a manufacturing company to scale, enter new markets, and pass inspections with confidence. It also builds stakeholder trust - from regulators to customers to investors - by demonstrating a clear commitment to high standards, safety, and accountability.
How AI is Enhancing Compliance in Manufacturing Industry
Human-led compliance systems, while foundational, often fall short in today’s fast-paced, multi-jurisdictional manufacturing landscape. With regulatory environments constantly evolving and production processes becoming more complex, manufacturers face increasing pressure to maintain consistent production compliance without delays, errors, or gaps. This is where artificial intelligence is proving transformative, introducing speed, accuracy, and adaptability that traditional compliance models can't match.
1. Real-Time Regulatory Intelligence
AI systems use natural language processing and machine learning to monitor thousands of global regulatory sources in real time - ensuring manufacturers stay current with changing laws, policies, and enforcement trends. Whether it's the FDA issuing revisions to 21 CFR Part 11 or new environmental requirements from the EU, AI tools can extract, summarize, and route updates instantly to relevant stakeholders within the organization.
2. Automated Documentation and SOP Validation
AI enables continuous cross-checking of internal documentation (SOPs, work instructions, technical files) against evolving standards like ISO 9001, EU MDR, or China's GB regulations. This ensures that production compliance remains intact across operations without depending solely on manual document control or periodic audits.
3. Predictive Quality and Risk Monitoring
By analyzing inputs from MES, ERP, and IoT systems, AI can identify anomalies in real-time - such as deviations in temperature, pressure, or operator behavior - and correlate them with potential compliance risks. This predictive capacity is especially vital in sectors like food and pharmaceuticals, where a single deviation can compromise entire batches.
4. Smart CAPA and Non-Conformance Workflows
Corrective and Preventive Actions (CAPA) and change control processes benefit significantly from AI’s ability to classify incident data, suggest root causes, and auto-generate action plans. This reduces cycle times and human error, helping manufacturing companies meet regulatory expectations around traceability and continuous improvement.
5. Competency-Based Training and Role Compliance
AI-enabled learning platforms can dynamically assess training effectiveness by monitoring performance data. They flag skill gaps and recommend retraining, ensuring that employees in high-risk areas (e.g., clean rooms, critical control points) remain fully qualified and compliant.
6. Localized and Multilingual Regulation Mapping
Manufacturing companies operating across regions must navigate complex, country-specific compliance environments. AI bridges language and legal barriers by translating and interpreting regulatory content in context - whether it’s BIS standards in India, COSHH in the UK, or ANVISA rules in Brazil - ensuring consistent interpretation and implementation.
The Role of Springs in Next-Generation Compliance
Our platform, Springs, combines all these capabilities into a centralized, AI-driven compliance hub - designed specifically for the manufacturing industry. From real-time regulation monitoring to automated audit readiness and multilingual support, Springs helps manufacturing companies scale their compliance operations without adding headcount or complexity.
Ready to transform your compliance strategy? Contact us and discover how Springs can future-proof your production compliance and reduce risk across global operations.
The Cost of Non-Compliance: Risks Every Manufacturing Company Faces
While AI and automation are revolutionizing how manufacturers handle regulatory tasks, technology alone doesn't eliminate the risks of non-compliance. If anything, as systems become more interconnected and data-driven, the stakes grow higher.
In the compliance-heavy manufacturing industry, lapses in production compliance can have serious financial, operational, and reputational consequences - especially for companies operating in multiple jurisdictions.
1. Regulatory Fines and Legal Liabilities
Government agencies around the world are tightening their enforcement efforts, making it clear that non-compliance will be met with swift penalties:
- United States: The FDA has the authority to impose civil penalties, seize products, and suspend operations for non-compliance with 21 CFR Part 11, cGMP, and other standards. OSHA violations can lead to cumulative penalties exceeding $100,000 in high-risk facilities. Ranbaxy Laboratories was fined over $500 million in aggregate after distributing adulterated drugs.
- European Union: Non-compliance with EU directives such as REACH (chemical substances), RoHS (electronics), and CE marking (product safety) can result in product recalls and market bans. Under GDPR, if production processes involve personal data and fail to meet standards, fines can reach up to €20 million or 4% of global turnover, whichever is higher.
- Canada: Under the Canada Consumer Product Safety Act (CCPSA), companies can be fined up to CAD 25,000 per day per violation. Health Canada enforces strict compliance for labeling, safety, and reporting. Pharmaceutical and food manufacturers are regularly inspected, with ratings that directly affect licensing. Failing to meet standards under the Canadian Environmental Protection Act (CEPA) can trigger investigations, product seizures, and forced recalls.
2. Recalls and Operational Disruption
Recalls are one of the most visible - and expensive - consequences of non-compliance in the manufacturing industry. They disrupt supply chains, trigger regulatory scrutiny, and erode consumer trust.
- In Canada, Health Canada estimates that recalls occur at a rate of over 1 per company annually in some sectors. In one notable case, a food manufacturer faced CAD 1.5 million in penalties for repeated labeling violations and non-compliant safety testing processes.
- In the U.S., a single food or drug recall can cost upwards of $10 million, not including indirect costs like PR crises and lost contracts.
- In the EU, manufacturers must halt distribution and notify authorities immediately if their products pose health or safety risks - often resulting in full market withdrawal across multiple countries.
3. Loss of Market Access
Even minor compliance failures can result in denied market entry or import bans:
- Canadian exporters targeting the EU must comply with REACH and CE marking directives under the CETA trade agreement. Companies like Dominion Colour Corporation were forced to urgently obtain REACH registration to maintain European market access.
- U.S. manufacturers have lost overseas clients due to missing ISO 9001 certifications or inadequate traceability protocols, particularly in the aerospace and medical device sectors.
- In Asia, failing to register with authorities such as Japan’s PMDA or South Korea’s MFDS can delay product launches by months, if not years.
4. Reputational Damage
In the manufacturing industry, credibility is everything. One major non-compliance incident can undermine years of brand-building:
- In Germany, a leading automotive manufacturer faced billions in losses due to emissions-related compliance fraud - reverberating across global markets and triggering government investigations in over 10 countries.
- In Canada, repeated inspection failures in a pharmaceutical plant led to not only product recalls but also long-term reputational damage that affected global partnerships.
Even if a company survives the fines, the damage to its brand often leads to lost contracts, investor pullback, and talent attrition.
5. Financial and Strategic Instability
The cost of non-compliance extends beyond fines and legal fees - it also weakens your company’s strategic position:
Small to mid-sized manufacturers in Canada spend between CAD 1,000–25,000 annually just on maintaining regulatory compliance. When those efforts fall short, the cost of corrective actions, third-party audits, and consultant remediation adds up fast.
Globally, compliance gaps often trigger unscheduled shutdowns, loss of certifications, and even legal disputes with suppliers or clients. Each of these outcomes can jeopardize key contracts and delay production pipelines for months.
Non-compliance is a risk to your entire operation. For every manufacturing company, ensuring production compliance means more than avoiding fines. It’s about maintaining market presence, sustaining growth, and building trust in a competitive and heavily scrutinized global environment.
Staying Ahead: Strategies for Ongoing Compliance in Manufacturing Industry
As the regulatory landscape continues to evolve and production environments grow more complex, staying ahead is less about reacting and more about proactively weaving compliance into every aspect of operations. In the compliance in manufacturing industry, sustained success hinges on strategic, systemized approaches that reinforce production compliance on a global scale.
1. Conduct Regular Compliance Risk Assessments
Maintain up‑to‑date awareness of your regulatory environment. Conduct periodic risk assessments that analyze current and emerging laws across each market you operate in - FDA in the U.S., REACH/CSDDD/CRA in the EU, Schedule M or China RoHS II, Health Canada, and CEPA rules in Canada.
- Use a centralized regulatory database mapping rules to specific products or facilities.
- Engage cross-functional teams (quality, legal, operations, procurement) to evaluate impact, likelihood, and mitigation strategies.
2. Formalize Policies, SOPs, and Compliance Objectives
Translate risk assessments into clear compliance objectives and structured documentation - SOPs, codes of conduct, audit guidelines - aligned with international frameworks like ISO 9001, ISO 14001, ISO 45001, HACCP, and GxP systems, depending on your sector and geography.
- Maintain version control and make documentation accessible across multilingual teams.
- Use defined templates for SOPs in regulated systems (e.g., FDA Part 11 controls, EU MDR).
3. Embed Training & Competency Tracking
Employees are your first line of defense for compliance.
- Provide role-specific training that covers relevant standards: GMP for pharma, HACCP for food, CE requirements for machinery, COSHH for UK chemical handling, and GAMP systems training for automation control.
- Track completion and effectiveness via LMS or learning modules integrated with QMS.
4. Implement Compliance Monitoring & Internal Audits
Regular monitoring builds resilience into production compliance workflows.
- Use process controls and automated compliance software to flag anomalies, adherence gaps, or outdated documentation.
- Perform internal audits periodically - risk‑based frequency depending on sector - mirroring PSM regulations (e.g., OSHA PSM audits every 3 years in U.S. chemical plants).
5. Build a Proactive Compliance Culture
Elevate compliance from a task to a core value:
- Set up cross-functional compliance teams or steering committees to oversee performance, metrics, and regulatory changes.
- Encourage reporting through safe, anonymous channels to catch issues early.
6. Leverage Technology & Data Analytics
Modern systems enable scalable production compliance:
- Integrated tools - ERP, MES, QMS - track audits, CAPA actions, change control, and training logs.
- Data analytics and AI-based compliance monitoring detect trends, root causes, and deviations before they escalate.
7. Establish CAPA and Continuous Improvement Processes
Non-conformance management is central to regulatory frameworks:
- Automate CAPA workflows, root cause analysis, and verification of corrective actions. Organizations adhering to FDA, EU GMP, or IATF 16949 standards benefit from this structured approach.
- Integrate audit feedback loops and analytics to evolve standards across sites and product lines.
8. Monitor Global Regulatory Trends and Adapt Quickly
Regulation isn't static - new mandates like the EU Cyber Resilience Act or updates under Health Canada can arrive with tight timelines.
- Stay connected: subscribe to agency alerts (FDA, MHRA, CDSCO, ANVISA, MIIT, etc.), engage with professional associations, and participate in standards forums.
- Review and update compliance plans regularly - at least annually or whenever a significant regulatory update occurs.
A manufacturing company that embeds these strategies into its daily operations turns compliance into an operational strength - not just a regulatory necessity. Proactive, integrated systems help maintain production compliance across borders and product lines, reduce risk, and foster sustained competitiveness in a global marketplace.
Conclusion
If production compliance once felt like a layer of bureaucracy, today it is something else entirely. It’s an active force reshaping how manufacturing companies operate, compete, and scale in a world defined by volatility and regulation.
The compliance in the manufacturing industry spans multiple dimensions - regulatory obligations enforced by governments, international quality frameworks that define operational excellence, and internal systems that align corporate vision with daily execution. Whether navigating FDA oversight in the U.S., meeting the sustainability thresholds of the EU’s CSDDD, adapting to Health Canada’s GMP requirements, or localizing systems for China RoHS, the expectation is not just to comply, but to anticipate.
For any manufacturing company, the question is no longer “Are we compliant?” but “Are we built to stay compliant tomorrow, next quarter, and three regulatory cycles from now?”
Real production compliance isn’t reactive. It’s built into processes, enforced by culture, tracked through systems, and adapted through intelligence - be it internal or algorithmic. And yet, the most advanced strategies still hinge on one critical factor: will leadership treat compliance as a growth lever or a cost center?
Every facility, every supply chain partner, every regional market carries its own regulatory context and risk exposure. The ability to harmonize those variables under a unified compliance architecture could determine who leads the industry - and who gets left behind when the next audit, breach, or product recall hits.
So the question stands: Is your compliance program prepared for what’s next, or just catching up to what already passed?

